Introduction
Cameron Croft:
Welcome everybody to our web series, Saving Money in Oil and Gas Operations. I am very excited today because we actually have a subject matter expert that’s going to come on talking about glycol dehydration units and performance optimization. We designed the series to talk to subject matter experts and bring them on and start sharing information because if you’re the smartest person in the room, you are in the wrong room. A little bit to get started, we got a little bit of housekeeping. Now, if you’re new to Zoom, throughout this presentation, there is a Q and A box. You can be typing in your questions throughout it and we’ll be trying to answer those questions throughout the presentation if you’ve got something specific. But we also have a Q and A section at the end. If you want to wait until the end, you can do that as well.
Cameron Croft:
Now, if something goes wrong, a lot of us are working from home, if you had your kids walk in, power goes out, something happens, don’t worry. What we’re going to do is upload this video to our YouTube channel and also our blog so you can be able to do a transcript and a search find if you’re looking for something in particular.
Cameron Croft:
My name is Cameron Croft. I’m the CEO of Croft Production Systems. Got my engineering and my master’s degree from the University of Houston and a black belt in Six Sigma so I believe in continuous improvement in utilizing data. But the person that everyone came to listen to today is Terry Nelson. Now what I like about Terry Nelson, I’ve known him for a few years and anytime that I have any issues or some curveballs that are happening, with his expertise and his years of experience, he’s able to tell me exactly what’s going on. He starting off on the offshore drilling rigs, kind of getting more interested in production equipment. Then in 1986, he actually started from the ground up, working on glycol pumps for a company called Flo Co out of Corpus Christi. And within 14 years worked himself up to vice president of that company then going on to NATCO working on with them for several years and then also Hanover.
Cameron Croft:
With Hanover, he was able to get his experience on glycol systems, aiming plants and working for them for 12 to 13 years. And then now he is single-handedly building this division with WPI and growing a what they’re focusing on. A lot of it is installs of glycol systems, commissioning, warranty work, repair work, and then overall troubleshooting and consulting. He is going to be our subject matter expert today, talking with us.
Cameron Croft:
He put this presentation together. He wanted to focus on the big topic highlights of sizing glycol dehydration units, the common questions that he’s seen over the years concerning glycol dehydration, and pretty well basic maintenance. I know everyone’s trying to save on cost right now, but either you pay for proper maintenance now or you’re going to pay for it later on. To kick off this presentation, I wanted to ask Terry, Terry, why do you want to be a part of this series?
Terry Nelson:
Well, it’s really a pretty good question. And I get asked that quite often and really it’s about information sharing. Our industry as a whole has always done a bad job of training our people in my opinion. Most of us are given a truck, a set of tools, and a map, and okay, go out there and fix that. And nowadays at least we have FaceTime and we have communication that’s a lot better than what it was in the early eighties. When I started, you were literally on your own.
Terry Nelson:
And what I find is when you get these young operators, technicians, engineers and you feed them information, they literally are like sponges and they appreciate it. And I look at it from process safety and operational efficiency. Whether they work for me or for an operations company or Croft or whoever they work for, we have to help these people be safe in their jobs and operate the equipment the way it was designed to be operated. So many times when we get someone with a performance issue, it’s not necessarily the equipment, it’s the way it’s being utilized or the way it was set up and commissioned.
Cameron Croft:
You believe sharing knowledge is that the best way of increasing the effectiveness and efficiency of the workforce?
Terry Nelson:
Absolutely. If we look at that, the re-concentration side versus the storage section, the burner firetubes are on the firetube side or the re-concentration side, that’s where the heat is. That’s where your burners are heating your glycol to your set temperature, say 380 degrees Fahrenheit. The glycol enters there, coming back from the flash gas separator and dumps into there. Then it spills over to the storage section and that’s where your pump gets its suction from. You fill your glycol from the firetube side, you get pump suction from the storage and you can see the two different levels there. And that’s critical because so many times, the only problem wrong with the dehy system is it’s too full. The level has gotten to be above the height of the weir and that spill over weir separates the storage section from the firetube side.
Terry Nelson:
One thing it does, it allows you 20 or 30 degrees of cooling between the two sides. Now the pump suction will be a little bit cooler. If you run it too high, you don’t have two separate sections anymore. You just have one section in the re-boiler and two things are going to happen. One thing is you don’t get the cooling because now both sides will be at the same temperature. And two, you eliminate vapor space, that empty space in the top of the reboiler is where you create steam. That’s where, when you flash off the water that’s entrained in the glycol. Because remember, we’re not cooking water off here. If we were cooking water off, 220 degrees it’d be fine. We’re flashing water off instantaneously. When the glycol falls into the reboiler through the steel column, the only water in the system is what’s falling in because the glycol that’s in the reboiler already is at 380 plus degrees.
Terry Nelson:
It’s already re-concentrated. It’s already 98 to 99% pure glycol. The only contaminants are what’s falling into the reboiler from the steel column. That’s why it’s critical to have that level and have some empty space there so we create that steam and that steam pushes that vapor out the steel column, the water vapor outlet connection and goes down the line to our VTEX recovery system or to a thermal oxidizer or whatever kind of environmental after control we have. But the level and reboiler is one of the most critical things. A good technician when he walks up to a dehy for troubleshooting, the first thing he’s going to check is the level glass in the reboiler for the color of the glycol and the height of the level in that system.
Sizing a Glycol Dehydration Unit
Cameron Croft:
Okay. All right. That’s when you’re talking about, reevaluating the sizing of your glycol dehydration, when it seems like everyone, no one wants to spend any money, but they’re also, they might be oversized and I knew you were putting a lot of pressure on them when I was talking with you. Yeah, can you explain this a little bit?
Terry Nelson:
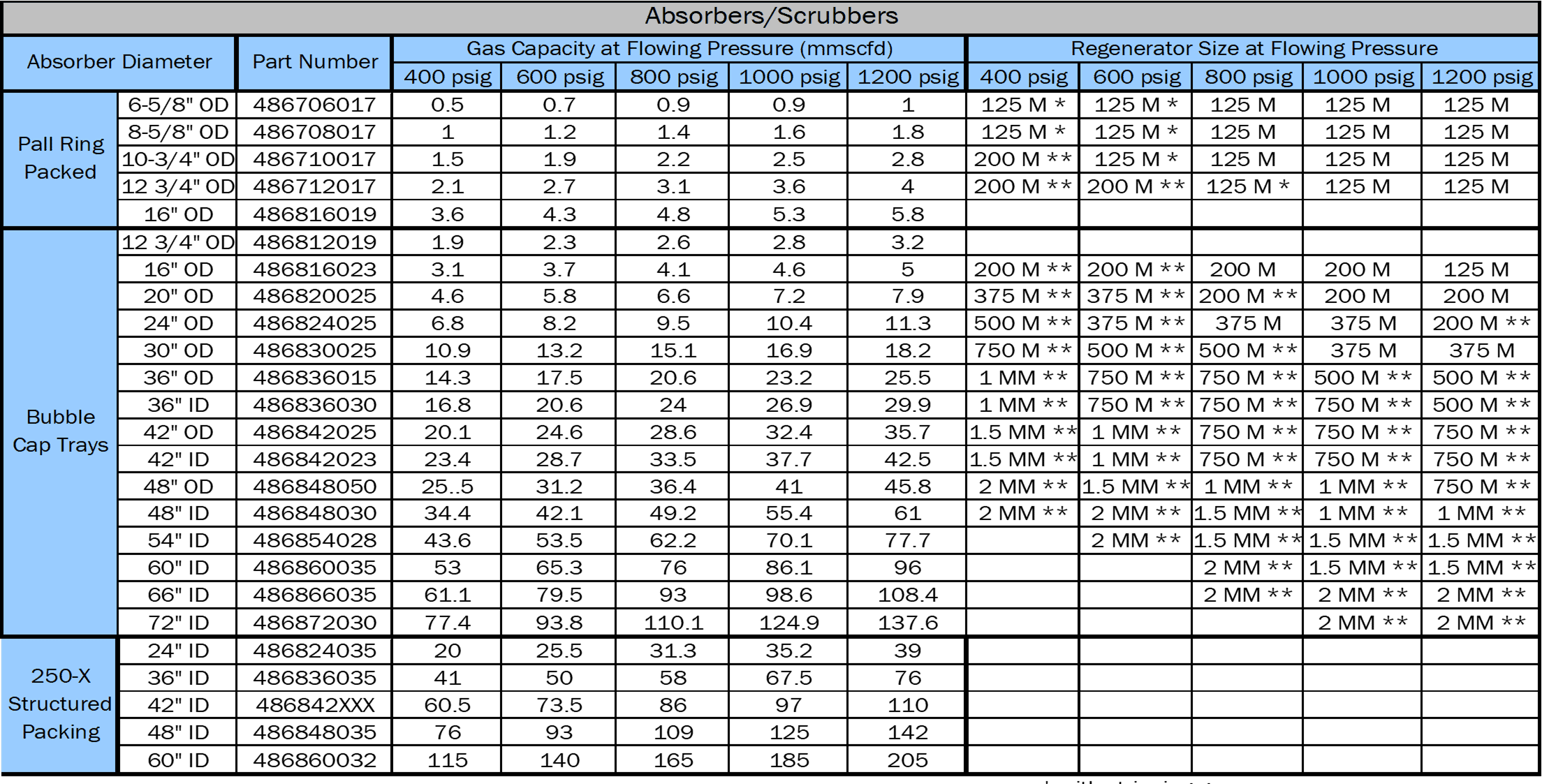
Absolutely. We all know that, a few years ago, and it wasn’t really that long, gas was $7, 7.50 a 1,000. And there was really aggressive push to build really large systems and create gathering stations where you might have a 60 inch tower or 54 inch tower. And you had multiple wells coming into one facility. You might have a tower that was good for 80 million cubic feet of gas. And over time, those wells start to play out and the flow rate starts to reduce. I was on location the other day, where it was originally sized for 46 million and they had 14 million. When you start looking at an oversized unit, you have to do a cost evaluation. It’s how much fuel gas am I using? The circulation rate that it takes to flood that tower with glycol.
Terry Nelson:
And you have to look at the number of trays or the amount of structured packing in it and what your glycol circulation rate is. Because remember, a tower might be designed for 50 million and you’re setting your circulation rate on that number. But if you only got 10 million going through it, you’re not going to flow through the tower as efficiently as you did before. You’re not going to have the surface contact area. And if you’re not careful, if you go down below what they call the turndown rate, then what will happen is the gas will channel through that tower and it will go through the tower untreated or not treated efficiently. A bubble cap tray tower has a five to one turn down.
Terry Nelson:
Whatever the maximum of that tower is when it was designed, 20% of it is your minimum target. If you got a unit that was good for 30 million cubic feet of gas at a set pressure, then a five to one turn down, once you get about six million, that’s your low end. If you get below that, then you’re outside of the efficiency zone and the gas will go through the tower untreated and cause other issues. A structured pack tower rather, has a 10 to one turn down. Twice the turndown rate through a structurally packed tower. I’m not talking about random packing like you would put in a little small tower, I’m talking about the actual stainless steel structured packing.
Cameron Croft:
Well, and we worked on a project before where together where they had the region sitting out there and it was good and the contact tower was good, but they swapped it out. And they think that just because they’re swapping the tower out to a smaller tower and it can handle the flow, the region couldn’t handle what they were wanting it to regenerate. And I like what, in the next slide, you broke it down into the steps of, this is how you determine if it’s proper size or not.
Terry Nelson:
Right. I was a product manager for Hanover/Exterran for many years. And one of my duties was to review sizing information when a customer would call in and they would want a particular, they would give us their extended gas analysis and determine what the flow rate was going to be. And we know what the pipeline contract is. To determine absorber size, you have to figure pressure variables because sometimes the pipeline pressure can change. And if the pipeline pressure drops, you still have to feed into that pressure. Sometimes we’ll put a back pressure valve on a tower so we can maintain a steady pressure so the fluctuations won’t affect the tower sizing. Some customers don’t want to do that. Then you have to let them know if the pressure drops 200 pounds, pipeline pressure, then the tower pressure also going to drop and that’s going to affect the tower sizing.
Terry Nelson:
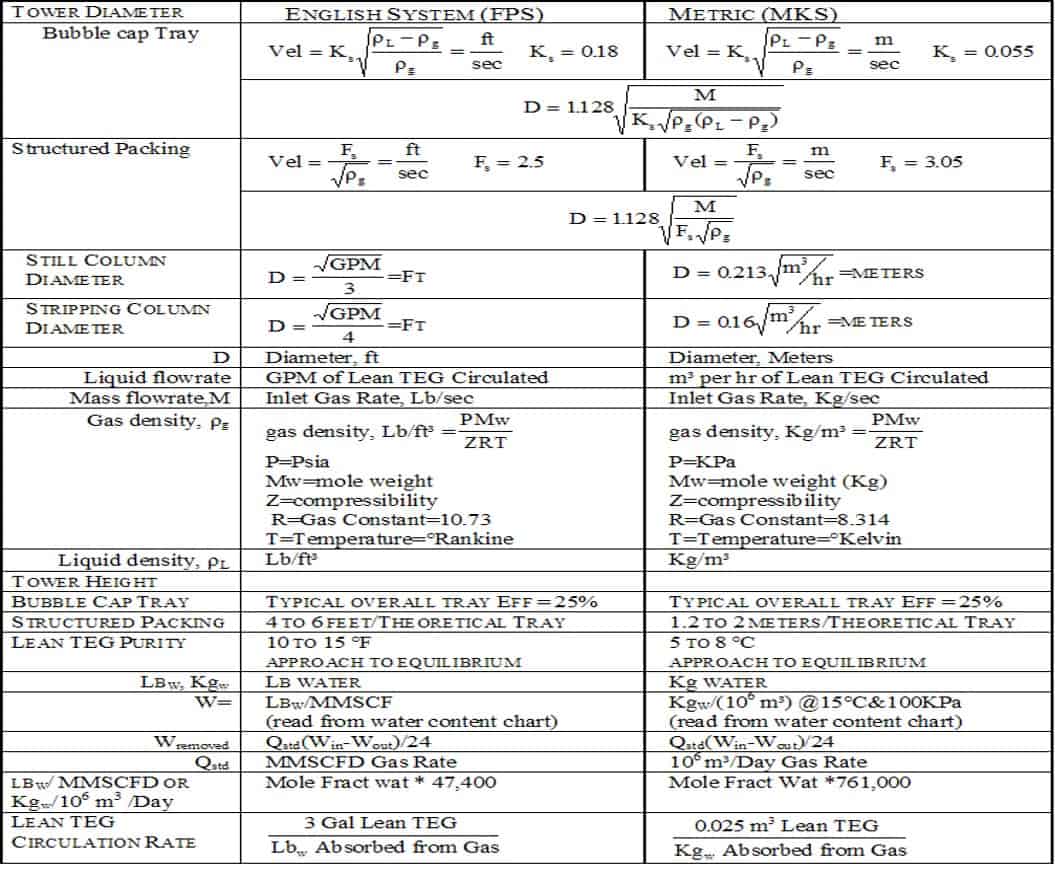
Sometimes wells pull back and we have to get them on compression to keep that pressure up. But the pressure is definitely going to affect the sizing. Then if we know what the flow rate is through the tower and if we know what the pressure and temperature of the gas is going to be, then we can determine the amount of glycol that has to move through that tower to drive the gas. If we have 1,000 pounds of pressure at 100 degrees Fahrenheit, then we’re going to have about 60 pounds of water per million cubic feet of gas coming into that tower. Now we know our pipeline is below the seven-pound dewpoint. That means we have to remove 53 pounds of water for every million cubic feet of gas. If we got 10 million cubic feet of gas, is 530 pounds of water we have removed.
Terry Nelson:
Next, that tells us the amount of circulation, because it takes about three to four gallons of glycol circulation to remove one pound of water. Now we know we take three times the 530, which gives us our circulation rate daily. Now we have to size our regenerator, our reboiler. When you say regenerator, you have to understand that means reboiler, pump gas separator, glycol pumps, and filters. That’s what makes a regenerator. A reboiler is part of that. A, we have to make sure our pumps are big enough to circulate that much glycol. We have to make sure that the pump gas separator or flash separator has enough retention time in it to absorb the glycol and let the hydrocarbons separate out. Our filters have to be large enough to handle that flow rate. And of course, our reboiler has to have enough BTU capacity to regenerate that glycol as it’s moving through the system.
Cameron Croft:
Well, I like what you said yesterday of the trays have a five to one turn down a structure has a 10 to one, but you’ve come across where the pumps, where some of your clients are operators. They know the pumps are so oversized that they’re wanting to change out all the pumps, the piping and they think it’s going to be this big project and you advised them of just putting a metering valve on the discharge side.
Terry Nelson:
There’s a lot of different ways that we’ve had to get creative through the years. And when you’re a service company, sometimes the best solution for the customer may not be the solution that makes you the most money, but it’s the right solution. You come up with something that helps your customer. And if that decision, a lot of times does not require you to tear that unit apart and downsize everything so that they can have lower flow rate when in a year or two from now they may add wells to that system and get the flow rate back up and then you’d have to change everything back.
Terry Nelson:
Sometimes you’ve got a really large pump, say it’s a Kimray pump and it’s a, let’s just say a 450-20 that’s maximum circulation rate is 400 gallons an hour. And when that unit was in full service, it needed that 400 gallons an hour to dry the gas. But now you’re in that turndown zone where you only need a very small portion of that. Maybe 75 gallons an hour or a 100. It’s hard for that big pump to slow down that much and work efficiently. What you can do is you can install a metering valve. It’s like a choke, almost like a needle valve in the discharge line between the pump and the contact tower. And you can open the speed control valves up on that Kimray pump and then control the speed of the pump with that secondary valve you installed. Now that bigger pump can stay on the skid, but you’re controlling the speed by metering how fast the glycol leaves that pump. Because when you think about it, the stroke of the pump is the emptying of one cylinder.
Terry Nelson:
By installing a metering valve downstream, you’re controlling how fast or slow that cylinder empties. You can reduce not only the speed of the pump way down below the stall speed, but you can also operate that pump at a much lower pressure than what the system or the manufacturers tell you that pump can operate at. I was telling you yesterday, Kimray will tell you, for instance, at about 250 or 300 pounds is the low end of the operational pressure for that pump. But I’ve got several dehys in the field right now that are operating just fine at 75 or 80 pounds of pressure by using this mechanism to do it.
Commonly Asked Glycol Dehydration Questions
Cameron Croft:
Well, that’s what kind of leads into your common questions. And I like what you were saying with the common questions is you’ve been changing this out over the year. One of your most frequently asked questions and issues that you have. Instead of me reading off all of this, we can dive straight into the nine common questions that you have.
Terry Nelson:
And like you say, I do change these quite often. Before the COVID-19 and all this happens, I traveled to the United States doing a four-and-a-half to a six-hour class on glycol dehydration and separation. Every class I teach, no matter if it’s an aiming system or a dehy system, I start with separation and controls. Because you have to bring the gas to process conditions so you can dehydrate it. A lot of the problems that come into a dehy system happened upstream at a separator or a filter set or a coalescer and that causes problems for the dehy. When I do my classes, at the end of it I hand out a scorecard so they can evaluate the class content, but they can also ask questions and I change out these questions as we go through.
Terry Nelson:
And these are the last few questions that I got. And the first one is the primary reasons that cause water saturation. When we look at water saturation when you talk about how much water is in the gas, you always first go to your chart and you look at pressure and temperature. But that’s a general guideline, but what causes saturation is usually something with the separator on the inlet side, or maybe a filter separator that didn’t dump properly and is carrying over and is flooding your contact tower with produced water. It could be an issue where I’ve got a guy on location right now in fact, and the dehy’s been working fine for three weeks. They called, something’s wrong with it. Well, we know how hot it is outside right now. July and August are always an issue because the ambient temperatures are causing in the gas temperatures to go up, which is causing our water load to increase. That’s what’s happening right now. We may have to adjust our circulation rates to accommodate the higher ambient temperatures.
Cameron Croft:
Yeah, it’s kind of hard to explain that with every 20-degree increase, you actually can double the amount of water saturation in that gas stream. It’s hard to see that curve the way it is.
Terry Nelson:
I tell my operators, I really don’t care what your gas temperature is at 8:00 o’clock in the morning. I want to know what it is at 3:00 o’clock in the afternoon.
And I want to set my circulation rate based on that higher temperature so that overnight and early morning hours you’re ahead of the curve. You’ll be really, really dry. But you’re prepared for that warmer gas temperature in the afternoon because so many of us now have gas moisture analyzers tied into our systems downstream of our dehy station. And when they get to a high dewpoint, they just start shutting the flow in. Well, we have to be prepared. We can’t be running out to the field adjusting pump speeds. We have to be prepared for that. Go ahead.
Cameron Croft:
Sorry. D
ealing with the controlling chlorides. The chlorides coming in with the water saturation, is that right?
Terry Nelson:
You always will have a little bit entrained in the gas, whether it’s.
Terry Nelson:
Have a little bit entrained in the gas. Whether it’s with the paraffin? Paraffin would bring a lot of salts and chlorides with it. But normally, yeah. It’s with a carryover of produced water. Because you always will have a little bit. And you take your quarterly glycol samples and you trim those out and you can see your chlorides rising. And that’s your best way of recognizing the symptoms is monitoring the levels of chlorides in the systems. And there’s not much you can do after the fact. You can clean the system, you can flush it. But you have to pull the fire tubes and inspect them. You can UT the fire tubes. And then, you track that information.
Terry Nelson:
But, yeah. Dealing with chlorides is tough. It’s usually a separation issue upstream when you’ve flooded the tower. The tower receives a lot of produced water. That glycol absorbs that. And that’s what black hall does.
Terry Nelson:
And then, the glycol makes its way back to the system where we cook off the water; we flash off the water. But those salts and chlorides are left behind on the fire tubes. And that leads to number three. I mean, you start getting a buildup of those chlorides on the fire tube.
Cameron Croft:
Yeah. So the bill? When it says, “recognizing symptoms of chlorides?” I mean, what would an operator look out for? Is there anything external? Or is it when he’s changing out filters and looking in the system itself?
Terry Nelson:
Yeah. You can spot it. Usually, you’ll start losing temperature in your reboiler.
Terry Nelson:
And what the chlorides will do is, they’ll build up a scale on the outside of the fire tube. And that will cause, that has an insulating factor on the fire tube itself. Your burner that’s inside the fire tube is transferring heat through that tube. And we have an illustration of that later. But that flame? When you get a coating of, whether it’s chlorides or other substances, on the outside of that fire tube, it will literally develop a patch.
Terry Nelson:
And I’ve seen it two inches thick. And that heat can’t transfer through the fire tube. So you create a hotspot, which accelerates the corrosion. But you’ll notice it. Because normally, your system is running 380 degrees and everything is fine. Now you start seeing your temperature starting to struggle to keep that temperature up. Like you said, you can spot it sometimes by your filters are plugging more often than they did before.
Terry Nelson:
You’ll also notice it in your glycol pumps. Because chlorides are an abrasive.
Terry Nelson:
And a Cameron pump has no lubrication on it. There’s not a grease fitting anywhere. It’s only lubricated by the glycol moving through it. So when you start putting something through it that has some abrasive or some grit to it, like a chloride or a salt, then you’re going to have some scratching and damage to the pump itself. And the pump will sometimes gas out or lose its prime. And usually, that’s because the scratching of the cylinders have caused a problem.
Cameron Croft:
Well, and your number four? I’ve actually never heard of an operator or any of our clients ever maintain or talk to us about pH levels. So I liked what you were saying here. So go on and talk about number four now for us.
Terry Nelson:
Usually, when I’m doing my classes, I’ll pull out a pH test strip. You know, I have fish ponds on my property. Aquariums? I’ve had aquariums through the years. All those things require pH control.
Terry Nelson:
Glycol also causes or requires pH control. Arrange a 6.5 or a 7 as a neutral on a pH scale. 6.5; anything lower than that, your glycol is literally corrosive. And anything over 8.5 is a problem because, after it gets any higher than that, your glycol will become an emulsion and it’ll start foaming drastically. So it’s important to… You can go to Home Depot or Lowes or anywhere. And for about $4, you can buy a plastic tube of pH strips. And it has a color scale on the back of it. And you dip that strip in some of your glycol. And I would suggest doing, on a rich test and a lean test, going to the tower and coming back. And do that pH test and hold it up against that color scale. And it’ll tell you exactly what the pH is. And in 30 seconds, any operator can tell what the pH of his glycol stream is.
Terry Nelson:
And if you’re losing glycol because of foaming? Which that’s the biggest reason. Then if you do a pH test, that’s going to be a leading factor on the troubleshooting to determine why we’re losing that glycol. I mean, this class is on cost-efficient use of operations of a dehy system. Number one cost is fuel. Number two is glycol losses.
Terry Nelson:
So this is where all this ties into.
Cameron Croft:
So your number five, “The chemicals to maintain proper pH?” Like if it starts going more acidity or corrosive, how would you go about treating that?
Terry Nelson:
Well first of all, let me just throw it out there. I’m not a chemical engineer. Most companies have chemical engineers on board. Now having said that, try ethanol amine. An amine solution, added to a glycol stream, will raise the pH of the glycol. So if you’re corrosive, you can add an amine. That will bring you back to the base side of it and get you a higher pH. The reason I don’t tell people to add much to glycol is because if I tell you to add a gallon and you add five gallons, you’ll go in the other direction.
Terry Nelson:
So that should be monitored. And also, anytime you add an amine to a glycol stream, you have to titrate that solution to know what percentage that you’re putting in it.
Cameron Croft:
Right.
Terry Nelson:
So that’s why you should always involve your supplier. If you’re an operator and you’re getting your glycol from whoever; Brenntag or whatever; Thomas Petroleum or whoever is supplying your chemicals? They usually will test it for free. They don’t advertise that, but they will. They’ll test it. And then, they’ll send you a glycol sample result where you can see what the different parameters, from chlorides to pH. And usually, that will come with recommendations, whether it’s adding a borax solution or an amine. And you can move that glycol up and down the scale, depending on what you add to the system.
Cameron Croft:
Well, if it goes to the other side of the base side and you start getting some emulsions and heavy foaming? I mean, is it a defoamer that you would add? Or what would you add to that?
Terry Nelson:
Yeah, defoamers are really a handy tool to have. Usually, all our service technicians carry defoamer on their trucks. We try to use a glycol-based defoamer. You have to be careful. Some of them are silicon-based and they can coat the inside of the towers. Especially, if you have a structured pack tower, it can affect how the packing works. So you have to make sure that…
Terry Nelson:
And you can take a sample of your foamy glycol in a beaker and put a couple drops of the defoamer of your choice in there and stir it around. And you can see how it affects the glycol solution and whether or not it’s going to be effective. And then, you know if you found the right defoam.
Terry Nelson:
I don’t advertise for anybody but I normally use a Baker product. It’s called Foam Break. It works really good for glycol. It’s just because I’ve used it for 40 years. And it’s just something that we normally use. Even on a large system, a half gallon is plenty. It depends on where you want to add it. Today, our systems are closed systems so we can’t open up a fill cap and pour glycol in, even though that might be the way we did it 30 years ago.
Terry Nelson:
But you can tie a connection into a pump suction and have the pump suck it right out. Or you can open a filter canister, bypass the filter canister, open the top of it, dump some defoamer into the suction filter, close it back up, put it in service, and the pump will suck it out of there and add it to the tower directly. It has an almost instant effect. Defoamer does.
Cameron Croft:
Well in your number six question, “Making adjustments to the stripping gas?” So what do you mean by adjustments?
Terry Nelson:
Well, a lot of people call it stripping gas.
Terry Nelson:
Some people call it sparger, a sparger gas. Depends on the design and the manufacturer. Stripping gas? Basically they have a piece of pipe that runs underneath the fire tubes with a lot of tiny holes drilled in it. Then you put about four pounds of dry gas onto that connection at about four pounds of pressure. And it’ll bubble up through that glycol. And that gas is dry. And the dry gas bubbling up through that glycol will absorb some of the moisture out of the glycol, giving you a stripping effect and helping you to get a little bit more purity in your glycol.
Terry Nelson:
On a sparger, a sparger box is attached to that spillover wier we talked about. It’s in the storage section attached to that spillover wier. And it’s a metal box that’s filled with ceramics; ceramic saddles or some structured packing; something to break up surface area. And then, the gas goes into that box and it bubbles up through that glycol as it’s spilling over the wier plate, giving you a little bit more purity.
Terry Nelson:
And we have to also understand that, if we’re adding stripping gas, we’re also increasing our emissions. Because that’s going straight out the steel column and it’s going to put an extra duty on the BTEX system downstream.
Terry Nelson:
So many, many times, more times than I care to admit, I’ve been on location. And that stripping gas connection has got 15 pounds of pressure on it. And they’re just roaring and rolling inside that reboiler. And it’s way too much. And they’re just wasting gas.
Terry Nelson:
And it’s all a zero-sum game in our business. Cost of processing is all added up and it’s all taken from sales. So everything we use for fuel gas, for stripping gas, waste gas off the pumps? All those things are added to the cost of processing. So we have to always weigh, is the cost worth what we’re spending?
Terry Nelson:
And so, any time, if we don’t need stripping gas, we don’t use it. And a long time ago, the stripping gas connections all had meters on them where you could actually measure how much gas you’re using for stripping. Nowadays, they don’t do that. There’s not a gauge there. So we have to really be careful with stripping gas so that we’re not just flooding the BTEX system with extra work to be done. Because all that gas, working its way through the system, goes out the steel column. And then, we have to deal with it downstream, either to a VRU or to a flare or to a BTEX system; whatever the case may be.
Cameron Croft:
Well, that’s what that, like the flash tank pressure adjustments?
Cameron Croft:
You’ve got to keep a minimum pressure. Well, I guess what is your rules of thumb on that?
Terry Nelson:
Well you’ll notice they’re all insulated, as well.
Terry Nelson:
Because the whole purpose of the flash tank? And when we say flash tank; glycol, condensate, separator. It’s got six or seven names. It’s just a low-pressure, three-phase vessel that’s part of the glycol reconcentration system. And what it does is, it really has three purposes. One thing it does, it separates the hydrocarbons out of the glycol stream. The second thing it does? It’s a wide spot in the line to let foam settle out. And the third thing it does; it captures the gas off the pumps, the flash gas. A ChemRate pump, for instance? It will create between three and five cubic feet of waste gas for every gallon of glycol you’re circulating, depending on tower pressure. So if you’re doing 100 gallons an hour, you can easily be creating 350 to 400 cubic feet of waste gas per hour.
Terry Nelson:
Now, most dehys have a mechanism where we can use that for fuel. And that saves us money. If you were in a cold weather situation, it’s not so much a good idea because that would be wet gas and it could freeze. But we can also send that to the tank battery through the condensate dump on that vessel and capture it by VRU and save that gas.
Terry Nelson:
Which is, we showed that slide earlier about circulation rates. I did a survey about; I don’t know, 20 years ago? And I checked 100 dehys. And we did major 20-, 30-point evaluations on them. Ninety-six of those 100 dehys were over circulating glycol, some of them at the rate of 30% or 40% more than what they needed to be circulating.
Cameron Croft:
Wow.
Terry Nelson:
So if you’re over circulating, then this vessel has more flash gas and it’s wasted.
Terry Nelson:
You said normal conditions. About 35 to 50 pounds of pressure, 160 to 180 degrees, will give you really good hydrocarbon separation. You know, when we billed as a separator, every vessel has retention time. A two-phase separator or a three-phase separator? You’ll have between two- and five-minute retention time built into those vessels. Pump gas or a flash tank separator will have about 20 minutes’ retention time. Reason because 35 to 45 pounds of pressure and a higher temperature will give you more retention time in that vessel for the hydrocarbons to separate.
Cameron Croft:
Yeah. Well, yeah. I liked what you were saying about efficiencies. Because the proper air/gas ratios for a burner? I know there are some huge efficiency losses there as well.
Cameron Croft:
And that’s your common question. So what do you like to see? What is a proper air/gas ratio for you?
Terry Nelson:
Well the important thing to know is, none of these come preset. When you get a unit to the field? Even if the manufacturer preset that air fuel settings on that burner? Just to drive out there with the road conditions and the bouncing around? It’s going to be moved around. So what I tell people to do? In part of the commissioning process, part of the burner lighting procedure should be to open that flame arrester up. At this point, the burner should be closed, the pilot should be isolated. It’s a natural draft system. So you open that flame arrester to get access to the burner. And you wait 10 minutes; 5 to 10 minutes. So you want to degas the fire tubes.
Terry Nelson:
So you open it up and you wait a few minutes; let the air replace any gas that might’ve been built up in the fire tubes. And then, if you notice, on this burner? There’s a primary air shutter. And then, there’s an adjusting pin for the main fuel. You screw both of those totally closed. Then, you back them off, one-and-a-half turns, and you lock them down. That’s a starting point.
Terry Nelson:
You know, when you talk about actual ratios, it’s going to depend on the BTU of your fuel and the pressure you set on it and all of that. But if you back those both out, one-and-a-half turns, and lock them down? And then, set your field pressure at 10 to 12 pounds of pressure and go through your lighting procedure and get everything lit and going? You should get a nice, blue flame with yellow specks on the end. And that’s what you’re looking for. That means you have a good fuel-to-air ratio.
Terry Nelson:
Now, once you do that and you close it back up, every burner system has a little window where you can look in and see your burner and you can see the burner flame.
Terry Nelson:
Once you close that system up and get everything lit, you can look in there and see if you have a good flame. And then, if you need to adjust it, you can. But if you walk up to a system and it’s just shaking? The burner’s going and the whole thing is just rattling and shaking? That usually means you have way too much air.
Terry Nelson:
And you need to idle back and close that air shutter a little bit to reduce the air flow.
Terry Nelson:
One of the things I tell people? On the troubleshooting side, if your system has always worked fine, no matter what it is; a heater treater or whatever; with the burner in it? And now, you seem to be getting some smoke in your stack or you’re having trouble reaching temperature? You have to sometimes do burner maintenance. We recommend it twice a year. And what we do is, we make it part of the preventative maintenance schedule.
Terry Nelson:
If you have some downtime on your location? Say you’re having a compressor worked on or your dehy cleaned out or whatever it is? And you’re shut down; process flow is down. You should have a list of 8 or 10 things that you want to check now that you couldn’t check before, because it would require a shutdown. Your burner is one of those things.
Terry Nelson:
And you also want to check your flame arrester on that burner.
Terry Nelson:
That’s where your air gets sucked into the burner. And in our conditions in South Texas, especially West Texas? A lot of sand, a lot of wind. That flame arrester will literally get plugged with sand. And you’re restricting your air flow to that burner. Take that flame arrester out, put it on the tailgate of your truck, and just pour a gallon of water across it. You will be shocked at the amount of sand that you get out of that flame arrester.
Terry Nelson:
And now, you’re going to get a much better air supply.
Cameron Croft:
So, Terry? We do have a question that popped up. It says, “What criteria do you use for determining when a dehy tower is likely to be maxed out for a given set of proposed operating conditions?”
Terry Nelson:
So I’m getting, I need to understand the question better. Normally, every manufacturer will be pretty close to the same. Because it’s all about velocity.
Terry Nelson:
It’s about how fast that gas moves through the trays or packing that are in those trays.
Terry Nelson:
Let’s look at a bubble cap tray tower first. A bubble cap tray tower has 8 to 10 trays in it. And the diameter of the tower determines how many bubble caps we can put in that tray. Trust me, they put every one they can get in that diameter to reduce the velocity across that tray.
Terry Nelson:
If you had a 36-inch tower but you only put six bubble caps in it, this gas is going to be screaming through that tower so fast, it’ll never have time to get treated. Now, you take that same 36-inch tower and you put as many bubble caps as you can fit in there? You’re reducing the velocity. The gas literally does bubble from one tray to the next. And in a bubble cap tray tower, it starts coming in the bottom. Every tray it goes up through the tower, it’s getting a little dryer on the gas. And the diameter is affecting how the gas moves is effecting how the gas moves through that tower velocity wise. Now, if the tower size for 30 million and just like it has a turndown rate on the minimum, it also has a maximum, because when you start crowding it, you’re increasing the velocity. You’re packing that tower and the gas is going to start channeling. It’s going to find a path of least resistance, and it’s just going to travel up through that tower without spending any time to be treated. That’s a bubble cap treat tower. Now a structured pack tower has two parameters, the gas rate and what I call, flooding. A structured pack tower is literally corrugated stainless steel packing, about 14 feet of it in that tower. The gas is going up through there, and of course, if there was no glycol flowing through it, the gas would just find a path and go out.
Terry Nelson:
Because it has a minimum circulation rate of glycol, you flood that packing with glycol, you’re creating the resistance. You’re holding that gas back, and that’s what creates the reduction in velocity, to let that gas go up at the current speed it needs to, to get dried. If you reduce that glycol below the flooding rate, then the gas will channel through the tower. That’s the way structured pack towers work. The biggest thing in a system where a customer has a tower that’s good for 40 million and he’s always put 40 million through it, and it’s always worked, but now it’s not working, what I find the biggest abnormal situations are, is the pressure has changed and the pressure has dropped. That’s going to be one of the variables that’s going to change the way the tower works, or the system is dirty. That’s usually the packing is dirty, the bubble caps are dirty. It used to handle 40 million, but now it won’t.
Cameron Croft:
Okay. Well, we got one more question that popped up. What I want to do, I think this is a good question towards the end, so I want to continue on.
Cameron Croft:
Okay. I know that people need to get off at noon, because people at lunch, don’t worry about that. We can upload the video. You can watch it later, but I’d like to keep going, because this is a lot of good information. Let’s go on to the maintenance for glycol, because like, what you were saying is people are starting to skip out on maintenance thinking they’re going to save money, but you’re going to pay one way or another, so it’s better to have proper maintenance. I’d like to go onto the first one that you had was thermal decomposition. Tell us about that.
Terry Nelson:
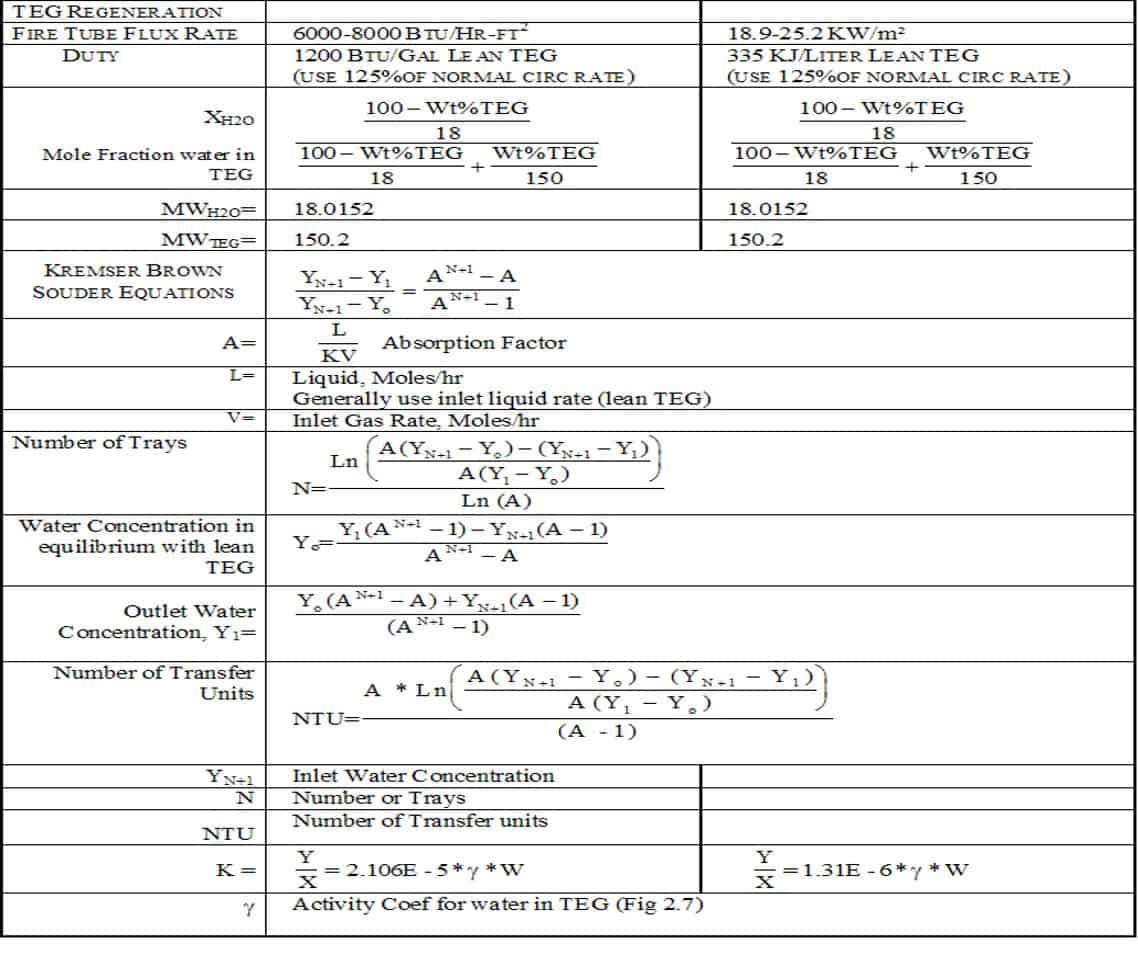
Well, thermal decomposition, triethylene glycol, when we’re talking about glycol dehydration, we’re talking primarily of triethylene glycol. Triethylene glycol boils at 456 degrees, but it starts to decompose at 405 degrees. We operate the system anywhere from 370 to 390. That’s the average operating range. The purity of glycol is based on the operating temperature of the reboiler. We want to keep it as hot as we can, but we also don’t want to cause any decomposition. What happens when you get glycol that gets decomposition, you start seeing an ethylene glycol to show up in the glycol samples and you lower your purity of triethylene glycol. We have to understand that dehydration is a mathematical equation based on the purity of glycol and how much we’re circulating. When we talk about maintenance of glycol, we’re talking about taking glycol samples.
Terry Nelson:
We want a rich sample and lean sample. We want to market with today’s date, rich or lean, and we want to test that glycol so that we can compare the rich sample to the lean. The rich sample tells us what’s coming in with our gas stream. The lean sample tells us how good our regeneration system is doing at cleaning up that glycol. Now, when we talk about this thermal decomposition, we also have to mention the localized overheating. When you look in your burner, when you open that flame arrester up, that burner should be dead center of the fire tube, so that you don’t have flame impingement where the main burner is running along the inside of the fire tube. Five or 10 degrees off either way will cause that burner to run down the side of the fire tube. That can cause some problems of overheating on the fire tube itself.
Cameron Croft:
Yeah. You kind of reiterated again, pH control, so this is also your maintenance. I mean, I know you were saying that was a common question, because I don’t hear anyone ever doing it, but you focus on it pretty strong if this is proper maintenance.
Terry Nelson:
I have a pump repair business as well in our company. Everywhere we function, we have a shop that repairs glycol pumps, and I probably do a thousand a year. The biggest problem we see is corrosion in the system. If you have a pH that gets below 6.5, your glycol is acidic. If you ever notice on a system where you have a glycol leak, when you have a glycol leak, glycol meeting air will become a corrosive. It will become an acid. If you ever notice where you have a glycol leak, you will have corrosion, sometimes on your steel column where the two flanges are mounted together. If the gasket leaks and the glycol runs down the neck of the steel column, it’ll eat the insulation up and you’ll get pinholes in your reboiler shell.
Terry Nelson:
You monitor your glycol samples, you’ll notice your iron content will start rising as your pH starts lowering. What that means is, the glycol has become acidic and you’re literally eating the iron from your equipment out, and it’s showing up in your glycol as iron. I mention it so many times because it’s a big problem in the system. If your pH gets above 8.5, your glycol will emulsify, and you’ll have a serious foaming problem in the system.
Cameron Croft:
Well, then salt contamination, so I know you kind of talked about it a little earlier about the chlorides entering your system. Inspecting that, I guess you said the telltale sign was that the system will take a little bit more to keep heating up to maintain temperatures. Is that right?
Terry Nelson:
Yeah. I see so many people that when the glycol really needs a physical cleaning, every dehy system, dehydration unit, should have a physical cleaning about every three to four years, where you just shut it down, pull the fire tubes, clean everything. When you do that, you inspect the fire tubes. You can UT it. You can map it out when you find a thin spot and you can monitor that, so you can do predictive maintenance and predict a failure before it happens. I tell people all the time, there are two kinds of downtime. There’s scheduled downtime and there’s unscheduled downtime. Scheduled downtime, you can reroute gas flow. Everybody knows you’re shutting down for cleaning. It’s good. That’s maintenance, that’s proper.
Terry Nelson:
Unscheduled downtime means that system failed. You have an abnormal situation, your gas is shut-in. You lose sales. Now you have to make repairs. The cost is different. I always tell people the cost of cleaning a system out is never the cost of cleaning the system out. It’s a cost of shutting the system down to clean the system. When we look at glycol samples, we use that as predictive indicators to track when that system is going to fail and then do something about it before it does.
Cameron Croft:
Well, you got this one picture you had. Is that chlorides or is that just a bunch of everything kind of getting burned onto the fire tube itself?
Terry Nelson:
Yeah, that is a function of not having a burner management system and not monitoring temperature safety high. No matter if you have straight thermostats controlling your temperature, or if you have a burner management system of whatever type, you have to have the temperature set in a place so that you don’t reach that 405 degrees. We have to remember that even though the reboiler is set at 375 to 380, the skin temperature of those fire tubes is about 800 to 900 degrees. It’s the liquid bath that keeps the temperature down. Once you get a lot of solids and hydrocarbons in the glycol stream, triethylene glycol might have a boiling point of 456 degrees, but hydrocarbons and solids are going to cake up and coke up your fire tube because they have a much lower boiling point and at 380 degrees, you’re creating solids. You can see in this picture. That’s why we like to pull the fire tube, so we can clean all that out and get the solids out of the system.
Cameron Croft:
This next picture, I was wanting to say this, we had the question come in, I’m going to ask her right now. He says, “We had a problem recently in our glycol reboiler. Its tubing got punctured. Due to the pandemic, we were unable to reach vendors, so we plugged that tubing, but all the other tubings are getting punctured as well within one to two days. The same happened in our other regeneration unit. Can you please tell us what are the major reasons for tubing punctures? The heating medium was a thermal fluid.
Terry Nelson:
Yeah, now, tubing, are we talking fire tubes, or are we talking something else?
Cameron Croft:
I think the way it sounded, let’s just talk about the fire tubes. Maybe that’s what he meant by that.
Cameron Croft:
Oh, shell and tube, that’s what he meant.
Terry Nelson:
Okay. Yeah. A lot of the heating mechanisms in these systems, or some of them are using hot oil, some of them are using the heat exchangers are the problem versus a fire tube. It’s all the same thing. It’s corrosion. If the glycol pH usually is too low, and you create issues because in a heat exchanger, you have a flow and they’re setting horizontal. Many times, they’re not flooded all the way full, and you have a flow pattern that develops, and you have a fissure created where the oxygen meets the liquid. That little layer where the air meets the liquid is where the corrosion occurs, and you get some erosion, just like you would in a lake bed.
Terry Nelson:
You can see where the liquid has flowed. The same thing will happen inside those exchanger tubes or in the shell side. As a liquid passes over there, you can get some erosion that occurs. I guarantee you, if you’re losing tubes at that rate, you need to check pH. You need to have an extensive glycol analysis done, probably because your iron content is really high. You may have to go to a different material for your tubes. Sometimes when we have welded tube bundles versus rolled, the welds are attacked, because they’re a dissimilar metal. That’s where the attack comes is in where the tubes are welded to the tube sheets. In this situation, this situation is a combination of things between high chlorides, high iron content and bad flame direction. You can almost see the damage on this fire tube is in the shape of a flame. That flame was running down the fire tube. Nobody noticed it, erosion occurred.
Terry Nelson:
Like I was telling you yesterday, we could take a piece of 12 inch pipe and throw it out on the ground and come back and look at it in a year. There’s going to be a little bit of pitting and erosion, but it would take years for that wall thickness to change. You put this in a glycol situation at 380 degrees and then have a hot spot where you’re not getting heat transfer, but heat transferring is occurring all around it. At the point where the patch is, that’s where the accelerated corrosion would occur. Just like in a tube shell bundle, under the liquid, not a problem. Above the liquid, not a problem, but where the liquid and the air meet, that’s where the ruptures occur. That’s where the corrosion occurs.
Terry Nelson:
I did notice, by the way, on the chat stream, when we were talking about maximizing tower flow, somebody said a sign of that, the first thing is glycol losses. If you start maxing the velocity out on your tower, in other words, you start crowding the capacity of the tower, the first thing you’re going to notice is tremendous glycol losses. That’s actually covered on one of our next slides, but I just wanted to say that before I forgot it. I did see that, but when you start maxing it out, the first thing you’re going to notice is increased glycol losses. Of course, you’re not going to make pipelines.
Cameron Croft:
What’s the typical glycol loss per cubic feet of gas that you’re processing?
Terry Nelson:
Yeah. You’re going to push gas into the sales line.
Cameron Croft:
You lose like a 10th of a gallon of glycol for every million or what is it?
Terry Nelson:
Yeah. The normal, and I hate to say normal glycol losses, because that’s a terrible term to use, but the understandable amount of glycol that we lose every day through a glycol [Dehy 01:02:32] system is one 10th of a gallon per million every day.
Terry Nelson:
If you’ve got a [Dehy 01:02:38] system processing 1 million cubic feet of gas, you’ll lose about a 10th of a gallon every day or about three gallons a month. 10 million a day, 30 gallons a month. A hundred million a day, you’re up to 300 gallons a month, normal losses through distillation out the steel column. There’s only four places we can lose glycol from, the top of the tower, and that’s going to go to the sales line, the bottom of the tower through the absorber scrubber, that’s going to dump to your slop tank, out the condensate dump and the flash gas separator, and out the steel column that would go to [B-tex 01:03:22] or to the thermal oxidizer. Those are the four places glycol can go.
Terry Nelson:
If we’re losing glycol beyond that 10th , we have to first identify where it’s going and why we’re losing it. Those questions we have to ask ourself. A guy called me a few weeks ago and said, “Terry, I need your help. Over the last month or so, I’ve lost 600 gallons of glycol.” I was like, “Man, you should have called me months ago.” Don’t wait till you’ve lost hundreds and hundreds of gallons of glycol to reach out for help. Glycol is $9 a gallon right now. We have to understand that’s a huge part of the cost of processing, so we have to address where we’re losing it at and how we can correct it. Glycol sampling is the key. It’s the key matrix for using our maintenance skills to find out why we’re losing it. I can tell you now, hydrocarbon content is a big reason for glycol foaming, as well as like one of the people said, “Maxing the tower out.” That’s a big one.
Cameron Croft:
Bill Jones has a question of, and I’m letting him know that we can kind of help him outside when this webinar is done, but they rotate a lot of equipment and they want to know kind of beforehand, ways of trying to figure out how, if it’s properly sized before it even gets out there. I know the parameters we always ask is, operating temperature, pressure, and volume. That way you can figure out saturation, you can figure out velocity going through the tower, what it can handle. Do you kind of do the same thing, you look for those parameters?
Terry Nelson:
That’s a big one, and that’s an important question, because just the fact that you’re asking it is a big deal, because so many people ask it after they’ve moved it and spent the money hooking it up, only to realize that it’s either oversized or undersized. Either way, it’s not a good deal.
Cameron Croft:
Yeah. It’s undersized, you’re going to see some carry over some more glycol losses than you need,
Terry Nelson:
You’re not going to have enough circulation. A perfect example. I recently had a customer had a reboiler that was 500,000 BTU. Originally that thing came with a 24 inch structured pack tower. It was sized properly and everything. Well, they decided just to change that tower out to a bigger tower, because they had more gas, available gas to flow. Should be fine just to put a different tower on that skid because it fit, but the problem is they didn’t any longer have regeneration capacity to dry that glycol out. You could change the pump out. That’s fine, but you don’t have enough fire tube flux rate or you don’t have enough burner space in the fire tube to create the heat, to regenerate the glycol at that circulation rate.
Maintenance Problems
Cameron Croft:
Well, your next maintenance problem was the hydrocarbons. I know you’ve been kind of talking about this the whole time, but hydrocarbons will cause major foaming issues.
Terry Nelson:
Absolutely. I mean, it’s the number one culprit. What I tell people is, glycol gas separator is your method of removal. When we talk about a regeneration skin, we have filters for particulates, we have heat exchangers for heat exchange. We have pumps for moving the chemical and we have a separator for removing hydrocarbons, and we have a reboiler for removing the water. All those things together create a re-concentration or a regeneration of glycol. That’s why it’s critical when you take glycol samples to take a rich and lean sample. I tell people constantly, “Anything you see in that glycol sample that’s not glycol, came in from the gas inlet with the gas.” When we take a lean sample, we compare it to the rich sample. When we take a lean sample and we still have 3% water, then we have to move to the reboiler and solve that problem.
Terry Nelson:
When we take a lean sample and we see three or 4% hydrocarbons, then we have to either go to our charcoal bed filters or to our separator, and find out what the issue is. If we take the lean sample and it has high particulates, then we have to address, are our filters the right micron, or are we changing them often enough. I used to have a trailer I drug around with me when I did these classes. I had cutaways in it and I circulated water through there to show the glycol flow, and I had air that would bubble through there to show the gas flow. People always said, “Man, I wish my tower had windows in it so I could see inside the tower.”
Terry Nelson:
Or had windows in it, so I could see inside the tower. That’s what glycol samples do, they allow us to look inside the unit.
Cameron Croft:
When you were talking about filtration, the other section was sludge. What do you mean by sludge going through the… I’ve seen it before.
Terry Nelson:
Yeah. I mean we always have solids in our glycol stream and they’re, oftentimes, tiny enough and they’re in suspension with our glycol and we don’t see it. If you have a 75 micron glycol filter, anything smaller than 75 micron is going right through. Over time, those particles will combine and get large enough to fall out of the flow. Sometimes we call them tar balls, and I’ve seen them as large as grapefruit. They get in the tower, they plug the trays, they plug the downcomers in the towers. They get under the reboiler, I’m sorry, under the fire tube in the reboiler. I’ve seen them get in the storage section of the reboiler where they plug off the glycol suction.
Terry Nelson:
What happens is, and you talk to the operators, and sometimes you take a filter apart to change it. It has black, sticky, almost tar looking stuff that’s on the filter. That is your suspended solid. With the tarry hydrocarbon, that has combined what we call the tar balls. Usually it’s because the pH has gotten low and there’s some corrosion going on. That’s where these solids are coming from mixed in with the dirt. You’re amazed how much dirt, physical dirt, is in a gas stream. It’s just amazing how much stuff that is in suspension with the glycol.
Cameron Croft:
You mentioned 75 micron earlier. Do you like that? Or do you like a smaller micron, like a 50 or so?
Terry Nelson:
Most manufacturers send 75 micron out with the systems because they expect some scale and different things in a system at startup. I usually like to drop down to a 50. I have customers that use 25 micron, but they change their filters every Friday religiously. You cannot drop down in micron size. I’ve had people tell me, “How often should I change my filters?” That’s a good question. The only answer is really a smart ass answer, which I’m good at, and those are when they’re dirty. You change them when they’re dirty. Your operator should know, if he’s a good operator, he’s going to know when his filters are dirty before he opens them up because he should be changing them pretty regularly.
Terry Nelson:
Monthly filter changes for glycol filters should be on the calendar. When you open that filter up monthly and it’s clean, then you either have a filter that’s bypassed or one with a micron size that’s too large. If you drop down a micron size, don’t wait a month because it’s going to get really dirty. The big difference between 75 and 50-micron filters. Good filtration will prevent sludge buildup. It’s also the number one thing to make your pumps last longer is good filter changing.
Cameron Croft:
You got sludge, good filter changing, your next one was foaming. I know foaming has got to happen one way or another, but you had a rule of thumb. I was talking with you the other day. You said foaming has a stability of 45 seconds until it turns to liquid, and that’s when you know you got at least some good foaming.
Terry Nelson:
We can take us a Mason jar or a beaker or something like that and take a sample of our wet glycol return coming back from our tower. We take a sample of that, it’s going to be foaming because it’s a mixture of gas and glycol. We take a sample of that and set it down, it should settle to a solid liquid in 45 seconds. Now, most of us that have worked on DHIs will tell you sometimes we’ll take that sample and it looks like shaving cream. It’s just thick and fluffy.
Terry Nelson:
Then, two or three minutes later, it still looks like shaving cream and it hasn’t settled to a liquid yet. If that’s the case, then you have lost stability in your glycol. You have no surface area and you’re probably losing it over the top of your tower. Your gas is not going to have time to have contact with that glycol in the tower because it’s foaming so much. It’s a good rule of thumb, check your pH with the litmus paper, and then also check your foaming with the foaming test. Just take a sample, set it down, hit your timer on your phone or your watch and see if it turns to a solid liquid in 45 seconds or less. If not, if you’re losing glycol higher than that 10th of a gallon per million, the first thing you want to check is the foaming because you see all the things listed there that causes glycol to foam.
Terry Nelson:
I have a lot of friends that work in the chemical industry and they tell me all the time, “Stop telling people that our chemicals make glycol foamy.” I always tell them, “Then why do you sell defoamer?” You notice that bottom one, high liquid vapor contacting velocities, that’s the person that asked about the tower being maxed out. When you have a tower that’s being maxed out, the first thing that happens is your velocity is going to cause you to start foaming and you’re going to start losing glycol.
Cameron Croft:
That’s good. No, that’s good. I know the other one, I’ve seen this to operators several times, is if something is not dehydrating properly, they immediately go to the reboiler and just kick it up. That or kick the pumps up or you kick the reboiler temperature, so yeah. Explain what you’re meaning here.
Terry Nelson:
I made this chart because I couldn’t find a good chart. A lot of the things in this presentation and I wrote a book on DHIs, and sometimes in these different slides, you’ll see where it refers to a page in a booklet. I wrote this book on DHIs because I couldn’t find enough information. I could find engineering documents, but I couldn’t find operational information about DHIs in the field.
Cameron Croft:
Did you publish that book?
Terry Nelson:
I have not. I’ve given thousands of copies away and I’ll be happy to share it with anybody that wants it. I give it away freely.
Cameron Croft:
Yeah. Do you mind the people that attended this, we can ship them a link to your contact? Or we could ship them a link to the book itself?
Terry Nelson:
By all means, anyone that emails me will get a copy of it.
Cameron Croft:
Okay. Perfect. All right. Yeah, we’d like to get on that.
Terry Nelson:
It’s 60 or 80 pages. It’s got all this information in, a lot of good just empirical data on DHIs as well as every time I would mash a finger or do something wrong, I would write it down. Through the years, I just made it a little cleaner and then I’ve put it into a booklet so that operators… And I never brag on myself, but I have a lot of people tell me it’s their Bible for DHIs. It’s one of my proudest moments when I have a young engineer that… I teach this class I do to a few universities, Lackawanna College in Pennsylvania and a few other ones I go and teach this class too. Then, I meet these engineers 10 years later when they’re working in the field.
Terry Nelson:
It’s a point of pride to me when they’ve learned some of their foundations by attending these classes. It’s a point of pride. This thermometer thing, I’ve seen guys operate DHIs as low as 360 degrees and dry gas because every gas stream is different, has a different BTU. You go to salt dome gas, it may have been dried when it was put in the ground so it’s not wellhead saturated gas. When we look at a gas chart, we’re talking about saturated gas but it’s not working efficiently. There’s a big difference between effective operation and efficient operation. Efficiency means you’re doing something and you’re using as little fuel gas as possible and you’re creating as little emissions as possible.
Terry Nelson:
People don’t realize we have to permit by rule in most states. In other words, they monitor your emissions and they charge you accordingly. We have to keep our emissions and fuel costs down. 375 should be the minimum temperature to operate a DHI system. At that temperature, you can only achieve purity of 97%. Now that seems pretty high, but at 97% pure glycol, you have to circulate five gallons of glycol to remove one pound of water from a gas stream. Now, remember 1,000 pounds of pressure, 100 degrees, 60 pounds of water per million. If you get one million cubic feet of gas, you got to circulate 180 gallons an hour of glycol at 99% pure. At 97% pure, you got to do almost 350 gallons an hour of glycol circulation. 385 to 390 you’re at 99%.
Terry Nelson:
When that DHI system was designed, engineered and sized, it was sized on 99% pure glycol. Actually I think it’s 99.6 is the number they use. As your glycol purity drops, your circulation has to increase, and that’s where you end up using more fuel gas and creating more emissions. Of course, 405 degrees, we talked about that with decomposition. At no time should that glycol temperature rise above there. That’s why my technicians carry a test thermometer in their truck.
Terry Nelson:
Six-inch thin, 550-degree thermometer because if they’re troubleshooting a reboiler in the field, they’re going to pull that existing thermometer out. They’re going to put a test thermometer in to ensure the accuracy of that thermometer. If your DHI unit in the field is 20 years old, the thermometer probably is 20 years old and it may have a spring that’s stretched, so we have to know that the thermometer’s accurate
Conclusion/ Q&A
Cameron Croft:
Absolutely. What I want to do, I don’t want to wrap it up. We actually got a few more questions, but what I want to do is go through our upcoming webinars. August 4th, we have Jesus Olivares talking about manufacturing and repairs, what to look out for, stress fractures out in the field, when to actually start doing some repairs. Then, August 18th, we have Fleaux Services talking about LACT units and meter runs and proper measurement and control and maintenance.
Cameron Croft:
If you’re interested in becoming a webinar speaker, if you know of someone, please reach out to us [email protected]. Like Terry said earlier, we really want to start sharing more in our industry and not just become effective but also efficient, as Terry likes to say. The only way we can do that is by sharing our knowledge.
Terry Nelson:
I attended one of the webinars you had a seminar a couple of weeks ago and it was really good. I had some internet issues, but I attended it for as long as I could before I got bumped out. It was really good. I really like these.
Cameron Croft:
That’s the one with the plunger lift?
Terry Nelson:
There we go, plunger lift.
Cameron Croft:
Yeah, Chad. Chad’s a fantastic guy, but he was an operator so he has that street advice and what’s going on and that expertise. On this, like you said earlier, we’d like to try to make these things better and better every single time. The only way we can do that is to have proper feedback. You will be getting an email and a pop up, please fill that out. We’ll ship you a shirt or a hat, in this case, Terry’s book, because we want to find that good information of how to make this information sharing better.
Terry Nelson:
Now we did see these last two questions, right?
Cameron Croft:
Yes. Yeah. I’m about to read them off right now.
Terry Nelson:
Okay, good. Okay.
Cameron Croft:
Our information, Terry and my information, is below. Reach out to us especially if you have any particular information, our engineering staff or WPI’s engineering staff and technicians will try to answer these questions. If you want a webinar from Terry specifically, he does these things all the time. I think he’s got three more today. What I’d like to do is start asking the questions. One of the engineers that was on it says, “We have seen a lot of rusting issues on the storage section area right above the sight glass on the reboiler. What can cause the area to rust on multiple units?” Is that that oxygen level that you were talking about?
Terry Nelson:
Yeah. Exactly. It’s a perfect example. I mean a lot of the old DHIs, you notice they have little ball valves sticking out. Just over halfway up on the sight glass, there’s a little ball valve that sticks out there. That ball valve will be exactly the same height as the spillover weir. That’s at 60%. What you’ll notice on that rust line, it’s usually right where the liquid and the air meet inside. That’s where the corrosion is maximized at that point. It’s going to be pH related. Most likely you’re going to see, if you take a pH, you’re going to see a pH of six, 6.5.
Terry Nelson:
We talked about things to correct pH. Just personally, I’m picturing that sight glass with the really dark glycol in it. Glycol should be clear to amber. When it starts getting hydrocarbon contaminated and pH starts dropping, you’ll start getting darker. I’ve seen it just jet black before. That’s going to tell you there’s a pH issue and your glycol purity has really deteriorated [crosstalk 01:25:15] high ethylene glycol.
Cameron Croft:
You’re talking about pH, this leads to our second question. It says, “I have an older DHI and we have a pinhole leak near the base of the still column. It feels like the base of the still column might be thinning out. Is this a pH problem? Or how do we stop the thinning from happening?”
Terry Nelson:
Glycol sampling, it’s too late now because it’s already occurred. The thinning is what happens is the steam is rising up through that still column. The low pH is causing a problem and it’s eating the metal in that still column. I’ve seen them where you could push them in with your finger and feel it flex. You got to realize that was quarter inch to three eighths inch pipe at one time. That’s how much it’s lost in wall thickness. If it’s doing it there, it’s doing it also in other areas of the reboiler under the insulation, just can’t see it. The fire tube needs to be pulled on that. You can clean all the sludge out from under it, then you can pull the still column off the flange and do an inspection.
Terry Nelson:
It’s very possible you can take some repad and repair and weld some repad on the outside of that still column, but you can’t do it in service because there’s too much hydrocarbon. It’s a fire hazard. You got to pull the fire tube, pull the still column, flush it all out, and then you can probably repair that metal and put it back in service. As far as stopping it, once you clean it all out, put new glycol back in it and you’re going to be back in service.
Cameron Croft:
Okay. You would advise just pulling it out, flushing the system, doing your repairs, what you need to do your repairs on, and then just by adding it, that might stop the issue from happening.
Terry Nelson:
It’s the condition of the glycol is probably gone past regeneration. It’s hard to say without seeing it, but if it’s already causing pinholes in the still column, I would imagine that you’re going to have to clean it, flush it, repair the metal. What you’re going to do is you have to peel back some insulation and you could probably cut the insulation back and then put some repad on that still column neck. Just take another piece of pipe, the same diameter, and cut you a section of it out and weld it to it thereby fixing the problem. It’s a low cost way to fix this problem. Then, put the unit back in service with fresh glycol, then monitor it, monitor that glycol so you don’t have the same problem another two years down the road.
Cameron Croft:
That’s good. That’s the end of the questions. I don’t see anymore questions, but we went 30 minutes over and I know you’re busy, Terry. I appreciate everyone actually attending. This is a lot of good information. If anyone out there has more specific questions, you can, again, reach out to me or Terry. Email, you can call in The Croft, we’ll figure it out and make sure we got the right people on board with Terry. Terry, I want to say thank you and thank you to WPI for coming on board and sharing your information with us.
Terry Nelson:
Man, anything I can do. Like I told you when we were talking earlier, this is a passion for me. It’s all about process safety and operational awareness and efficiency. Any way I can help, you can reach out to me either on LinkedIn or by email or you can contact me through Croft. I’m more than happy to help in any way I can.
Cameron Croft:
All right, Terry. I appreciate everything and thank you for everyone who attended. We’re going to go ahead and end this webinar. Thank you again.
Terry Nelson:
Thanks guys.